转自: 科研者言(微信公众号)
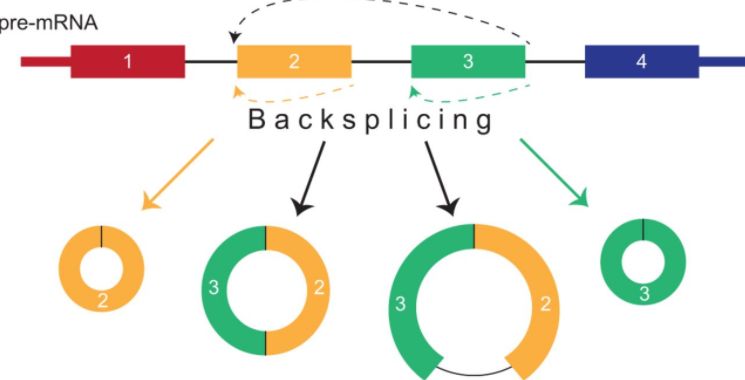
今天给大家说说circRNA吧,就是环状RNA,环状RNA的定义是什么?circRNAs(Circular RNAs,环形RNA分子)是一类不具有5' 末端帽子和3' 末端poly(A)尾巴、并以共价键形成环形结构的非编码RNA分子。它们通过pre-mRNA在细胞核内,
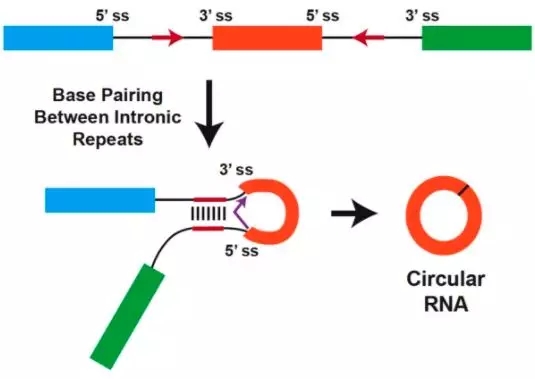
与之相对的,就是具有5' 末端帽子和3' 末端poly(A)尾巴的RNA分子了?那是什么呢?就是mRNA、lncRNA这些两端开放的RNA分子了。
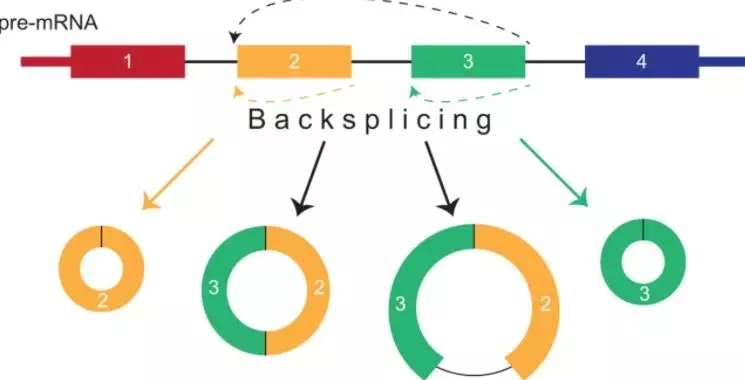
circRNA在发表的高分文章中可大致分为三类机制:ceRNA(吸附miRNA,作为海绵)、可编码翻译成为短多肽、结合功能蛋白调控其胞内功能。
而在国自然基金申请方面,项目也逐年增多,看看2018年申请到了多少个呢?如下
看的出来,8成以上的机制都是作为ceRNA机制调控miRNA的水平,参与调控下游靶基因的表达和功能。
那么我们如果需要创新的话,从已发表的文章中汲取好的思路,所以今天我们给大家带来3篇circRNA的高分文章,供大家学习和消化。
1.Exosomal circRNA derived from gastric tumor promotes white adipose browning by targeting the miR-133/PRDM16 pathway.
Int J Cancer7.361区4. 2019 May 15

分子机制亮点:不仅仅是ceRNA,还有热点外泌体。本文通过对胃癌病人的血液外泌体进行测序,结合统计学分析发现ciRS-133和白色脂肪组织变成褐色脂肪组织有相关性。通过进一步的细胞培养和测序发现,胃癌细胞可分泌携带了ciRS-133的外泌体(exosome),在体外和体内功能实验中发现,敲减掉ciRS-133的脂肪细胞前体细胞,可抑制其向褐色脂肪细胞分化。分子机制上,ciRS-133通过吸附抑制miR-133,促进了靶基因PRDM16的表达,在这个代谢方向里,PRDM16水平升高可使白色脂肪转为浅棕色脂肪是一个经典知识点,所以这篇文章通过将上游创新点结合明星基因PRDM16,形成了完整的创新机制。
背景:恶病质(cachexia)亦称恶液质。表现为极度消瘦,皮包骨头,形如骷髅,贫血,无力,完全卧床,生活不能自理,极度痛苦,全身衰竭等综合征。多由癌症和其他严重慢性病引起。可看作是由于全身许多脏器发生障碍所致的一种中毒状态。此症的发生多指机体处于严重的机能失调状态。
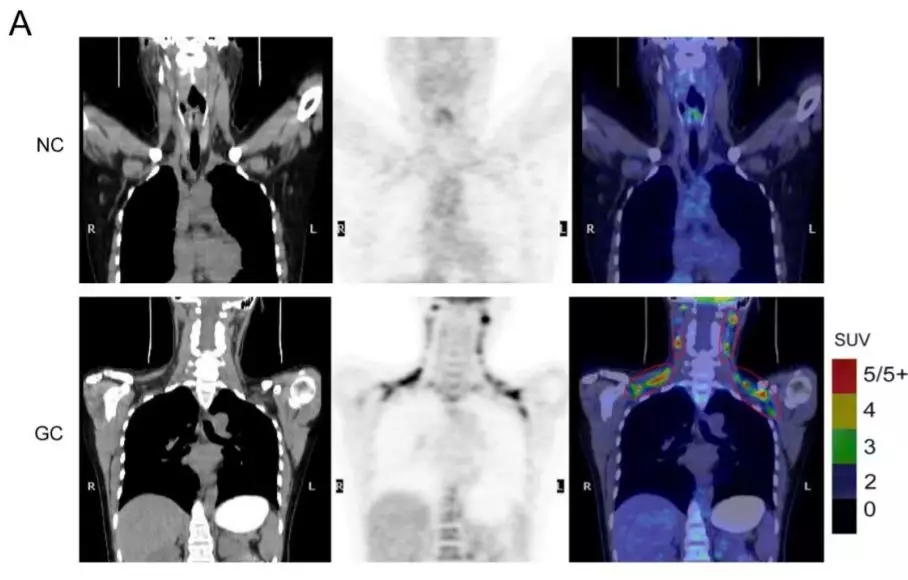
英文摘要:Cancer-related cachexia.The treatment options for cancer cachexia are limited, and the molecular mechanism remains poorly understood. Exosomes are small vesicles derived from cells.In our study, we showed that circRNAs in plasma exosomes have specific expression features in gastric cancer (GC), and ciRS-133 is linked with the browning of white adipose tissue (WAT) in GC patients. Exosomes derived from GC cells deliver ciRS-133 into preadipocytes, promoting the differentiation of preadipocytes into brown-like cells by activating PRDM16 and suppressing miR-133. Moreover, knockdown of ciRS-133 reduced cancer cachexia in tumor-implanted mice, decreasing oxygen consumption and heat production. Thus, exosome-delivered circRNAs are involved in WAT browning and play a key role in cancer-associated cachexia.
2.Translation of the circular RNA circβ-catenin promotes liver cancer cell growth through activation of the Wnt pathway.
Genome Biol13.2141区1. 2019 Apr 26
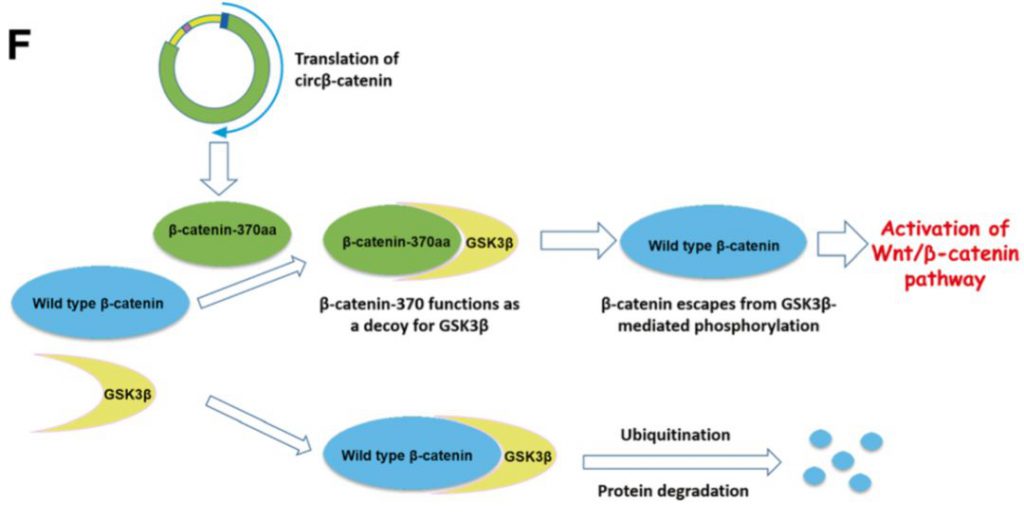
分子机制:首先检测发现Circβ-catenin是一个更多分布在细胞质中的一个circRNA,尤其在肝癌组织中高表达。敲减掉Circβ-catenin后,可在细胞内和动物体内抑制肝癌细胞的增殖以及肿瘤的大小,抑制其生物学恶性行为,而在信号通路的检测上发现其和β-catenin的表达正相关,但是不改变其mRNA水平,只改变其蛋白水平。但发现Circβ-catenin可编码多肽,命名为β-catenin-370aa多肽,这个多肽是β-catenin同型异构体,也就是结构上类似,经过测序发现Circβ-catenin其实是β-catenin基因转录本环化形成的circRNA,那么这个多肽即可结合GSK3β,抑制其磷酸化β-catenin以及泛素化降解的经典途径,这是另类的牺牲自己,拯救兄弟的机制。
英文摘要:Circβ-catenin is predominantly localized in the cytoplasm and displays resistance to RNase-R treatment. We find that circβ-catenin is highly expressed in liver cancer tissues when compared to adjacent normal tissues. Silencing of circβ-catenin significantly suppresses malignant phenotypes in vitro and in vivo, and knockdown of this circRNA reduces the protein level of β-catenin without affecting its mRNA level. We show that circβ-catenin affects a wide spectrum of Wnt pathway-related genes, and furthermore, circβ-catenin produces a novel 370-amino acid β-catenin isoform that uses the start codon as the linear β-catenin mRNA transcript and translation is terminated at a new stop codon created by circularization. We find that this novel isoform can stabilize full-length β-catenin by antagonizing GSK3β-induced β-catenin phosphorylation and degradation, leading to activation of the Wnt pathway.
3.Circular RNA cSMARCA5 inhibits growth and metastasis in hepatocellular carcinoma.
J Hepatol14.9111区30. 2018 Jun

分子机制:这个分子机制还是ceRNA,但是是双miRNA,也就是一个circRNA可吸附两种miRNA的机制,在课题上也算是创新的一种。通过高通量测序,分析得到cSMARCA5在肝癌组织中呈现低表达,其被高表达的DExH-Box Helicase 9抑制,通过独立的分享因子分析,低表达的cSMARCA5肝癌换着预后更差(OS、RFS都差),肿瘤的侵袭性更强,体内体外实验发现高表达cSMARCA5之后肝癌细胞的增殖和侵袭能力变弱,机制上发现原来其可同时吸附miR-17-3p and miR-181b-5p两个miRNA,阻止它们靶向降解TIMP3蛋白(百度一下该蛋白,会发现其属于一个抑癌基因,明星基因)
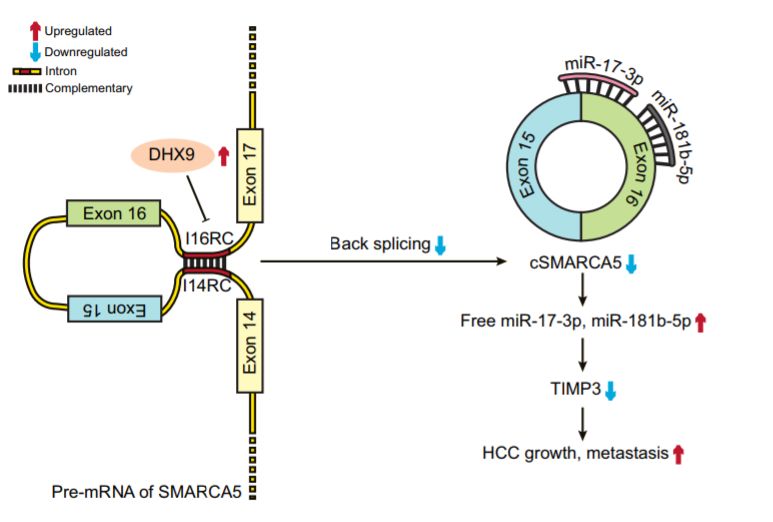
英文摘要:The expression of cSMARCA5 was lower in HCC tissues, because of the regulation of DExH-Box Helicase 9, an abundant nuclear RNA helicase. The downregulation of cSMARCA5 in HCC was significantly correlated with aggressive characteristics and served as an independent risk factor for overall survival and recurrence-free survival in patients with HCC after hepatectomy. Our in vivo and in vitro data indicated that cSMARCA5 inhibits the proliferation and migration of HCC cells. Mechanistically, we found that cSMARCA5 could promote the expression of TIMP3, a well-known tumor suppressor, by sponging miR-17-3p and miR-181b-5p.

来第一个抢占沙发评论吧!